The chapter described in the following post is part of a very graphical powerpoint presentation on soil microbiology. This presentation is freely available at the Helmholtz-Centre for Environmental Research (UFZ) in Leipzig, Germany.
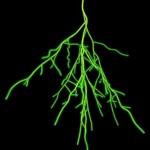
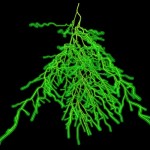
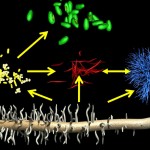
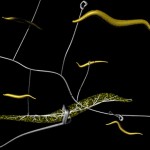
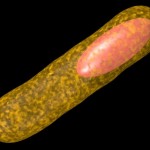
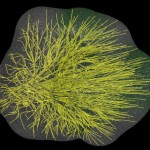
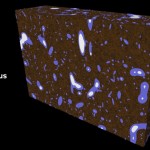
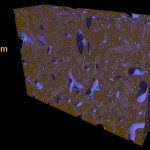
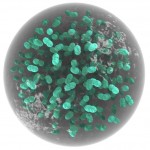
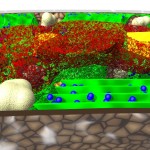
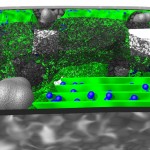
The title of chapter 5 is “Symbioses” which refers, to put it more precisely, only to mutualistic interactions between plants and microorganisms. There are a lot of such mutualistic interactions below ground. The reason for this is the fact that plant roots have a lot to offer to microorganisms. Plant roots are continuously excreting organic substances, which can be used as nutrients by microorganisms. This way they are like oases in the soil desert. The following image shows (in green color) the space influenced by the exudates from a small root system, the rhizosphere.
- Rhizosphere
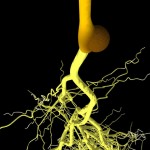
Microorganisms supporting plant growth increase the size and quality of this rhizosphere (their habitat).
- More roots translate into a bigger rhizosphere
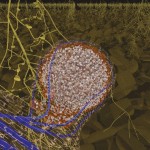
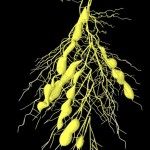
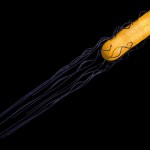
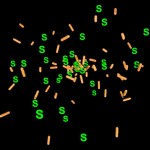
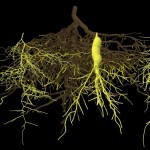
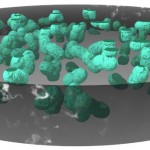
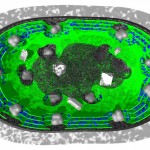
Many bacteria have chosen this approach. Some are producing growth factors, others are combating plant pathogens and others again are solubilizing or producing mineral nutrients. An example for this latter group is rhizobia, which are fixing atmospheric nitrogen. The process of nitrogen fixation is most efficient in the enclosed environment of the root nodules which contain transformed bacteroids devoted exclusively to satisfying the plant nitrogen demand.
- Root nodule
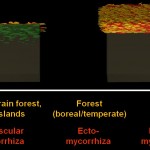
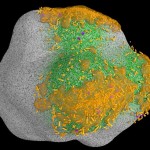
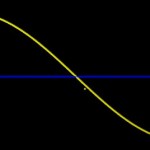
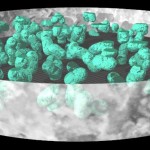
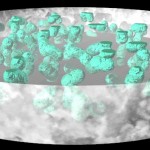
While bacteria can bridge only very short distances, fungi have the capacity to work over long distances, to explore the soil and to transport nutrients from various locations. Since they do this more efficient than roots (given their much finer hyphal system), mycorrhizas (symbioses between roots and fungi) have been very successful in evolution. These symbioses (arbuscular mycorrhiza, ectomycorrhiza, ericoid mycorrhiza) have already been covered in earlier postings. The following image shows the distribution of such symbioses, according to their ability to liberate nitrogen from organic remnants on a gradient of inhibition of plant litter degradation in various ecosystems.
- Mycorrhizal ecology