 Aldehydes
Aldehydes
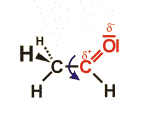
The aldehyde group is formed by an oxygen atom connected to a carbon atom via a double bond. In contrast to ketones, the respective carbon atom is connected to at least one hydrogen atom.
The structural formula of an aldehyde containing two carbon atoms (acetaldehyde) is given as an example. The influence of the oxygen atom leads to a partial positive charge of the carbon atom – it easily reacts with other groups. Due to the double bond, the oxygen atom, the carbon atoms and the hydrogen atom belonging to the aldehyde group are within a common plain. The covalent bond connecting the two carbon atoms, in contrast, can rotate freely. Accordingly, there are many possible structures; the structure shown gives only one of these possibilities.
Aldehydes are positioned between the oxidation levels of alcoholes and carbonic acids. They are formed by the oxidation of alcoholes or by the reduction of carbonic acids. Thus, acetaldehyde shown here can be produced from ethanol or from acetic acid.